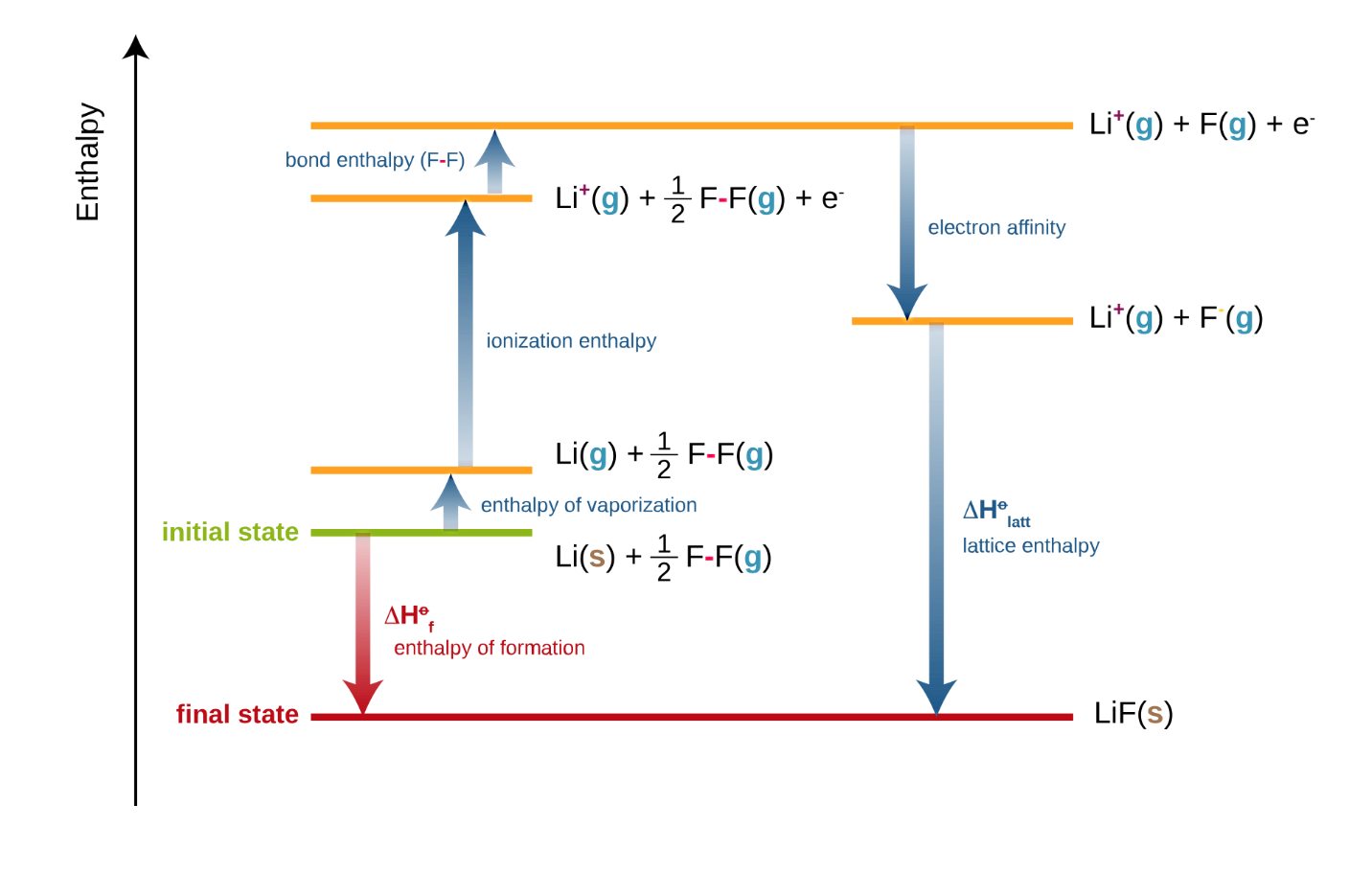
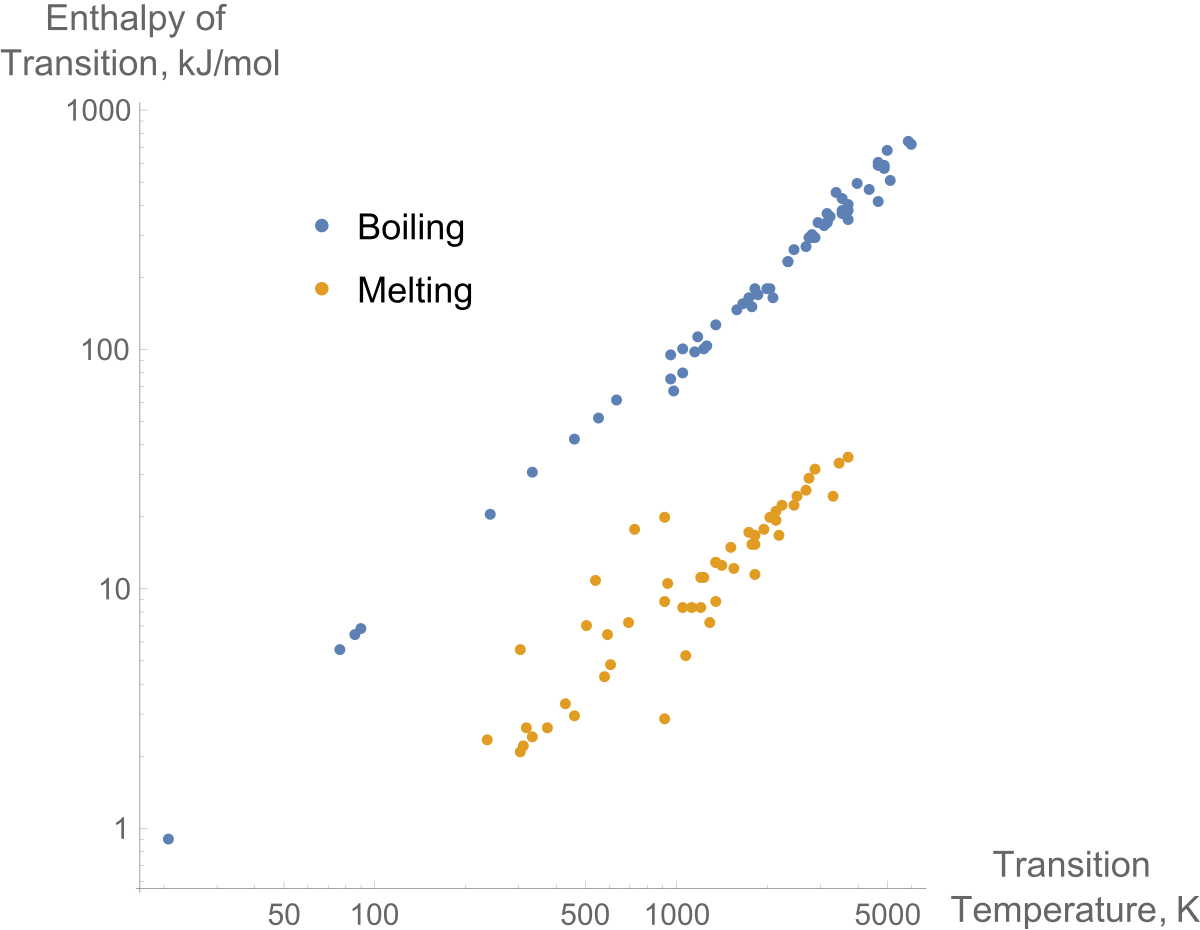
Enthalpy of vaporization vs. temperature for the truncated and shifted... | Download Scientific Diagram

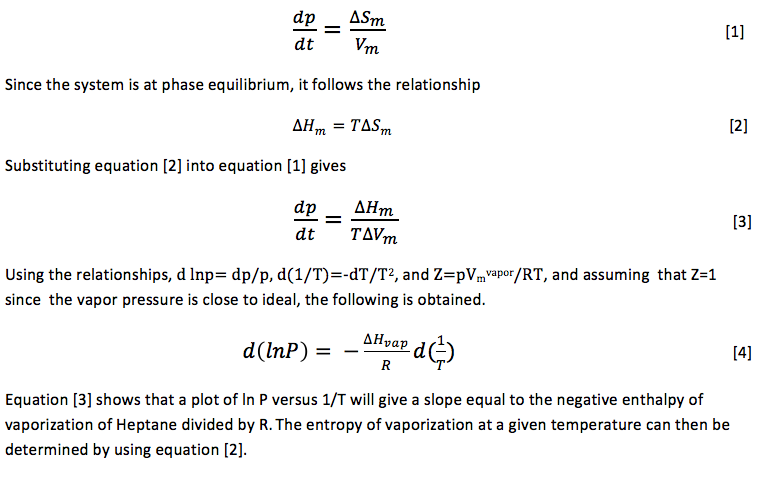
SOLVED: 7 The enthalpy of vaporization of water at 100.0-C is 40.68 kJ mol. What is the entropy change in the surroundings when one mole of water vapor condenses at 100.0 %C

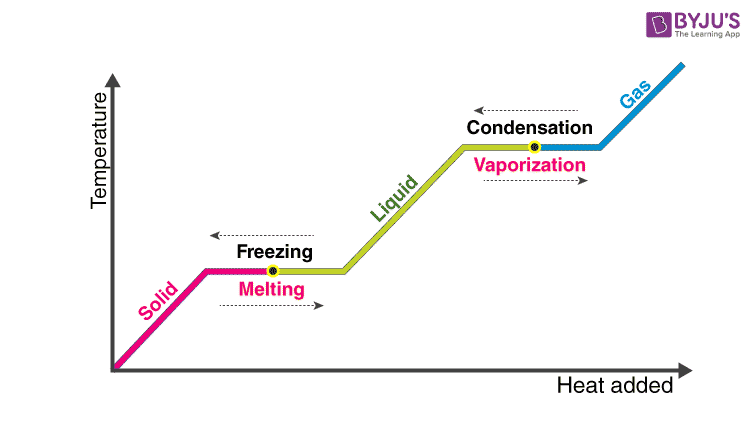
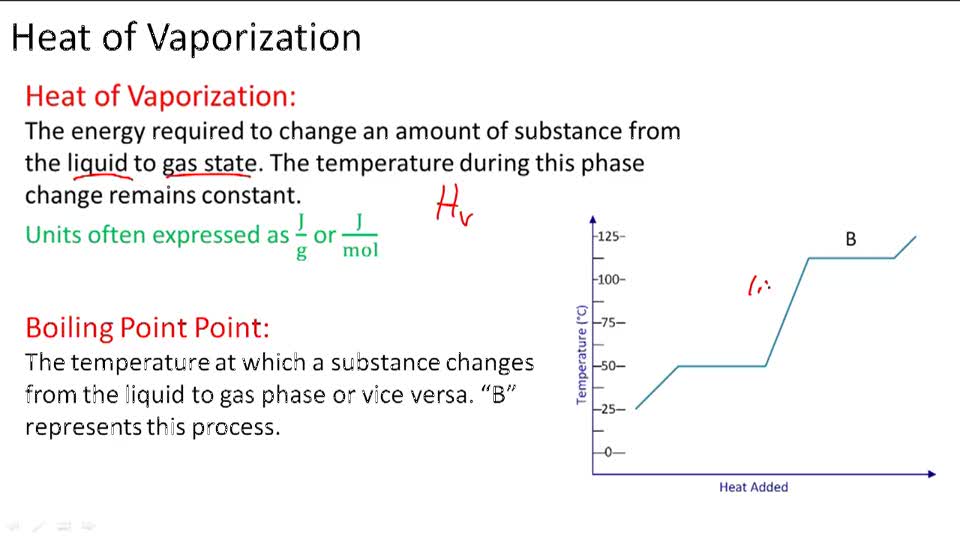
11.3 Heat in Changes of State. Warm up Is it exo- or endo- thermic???? - negative ΔH -positive ΔH -Heat as a reactant -Heat as a product -Combustion of. - ppt download

Intermolecular Forces In the particles of a liquid the particles are much closer than in a gas. This is because they have greater intermolecular forces. - ppt download