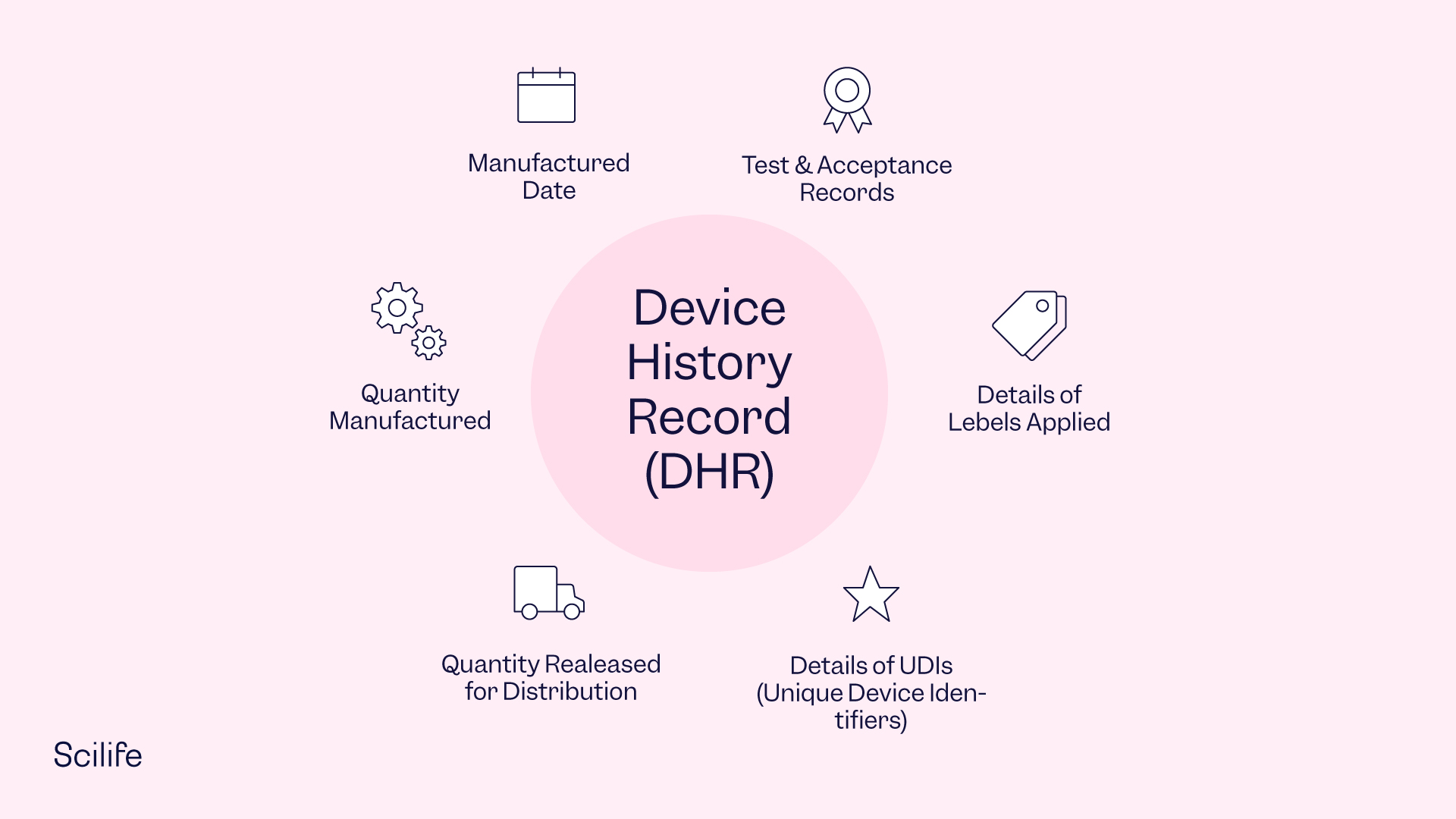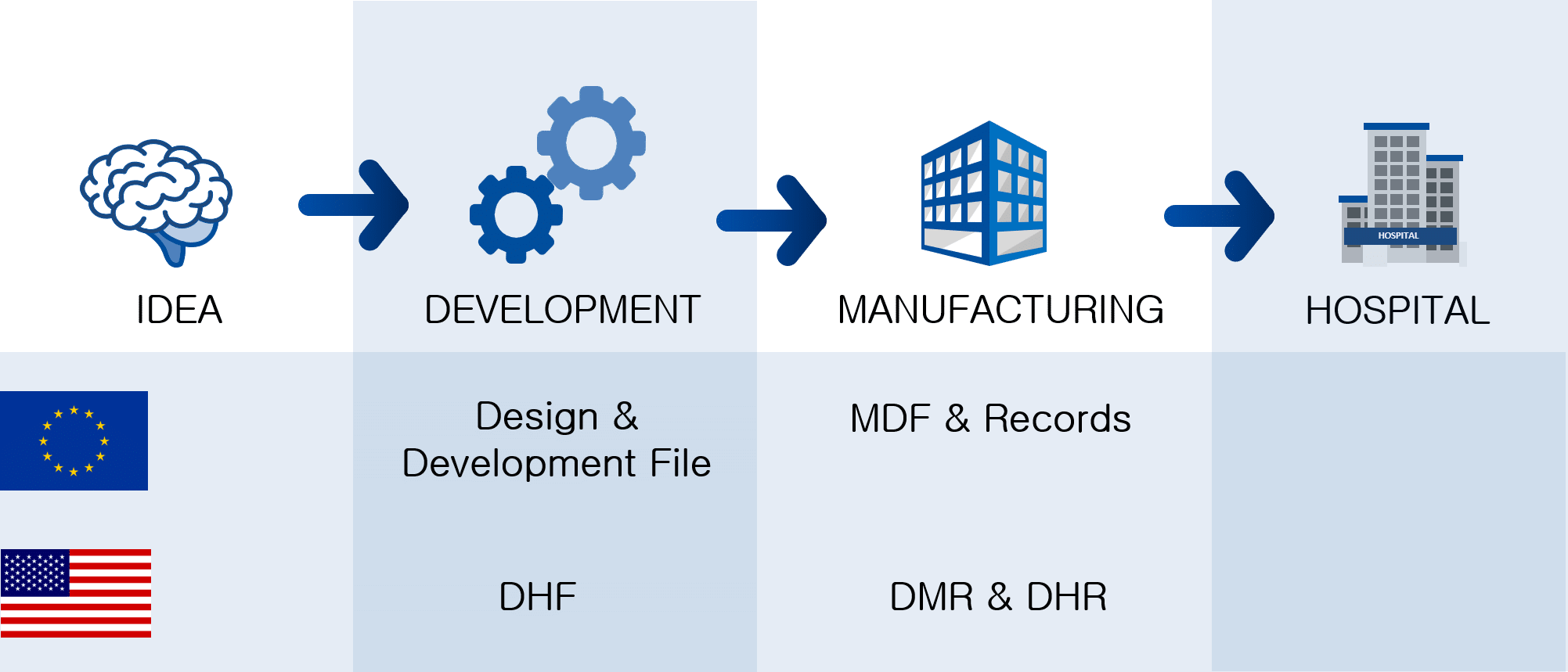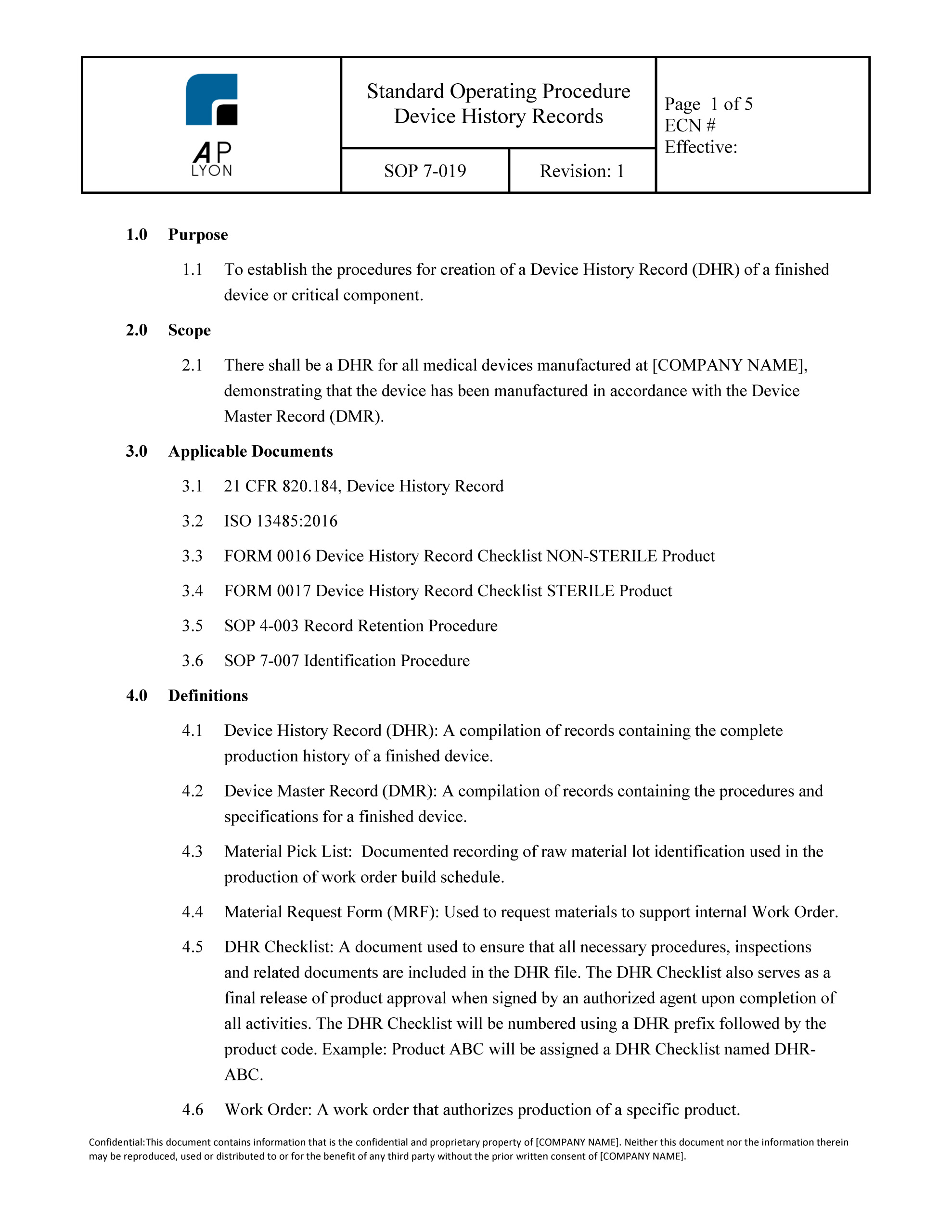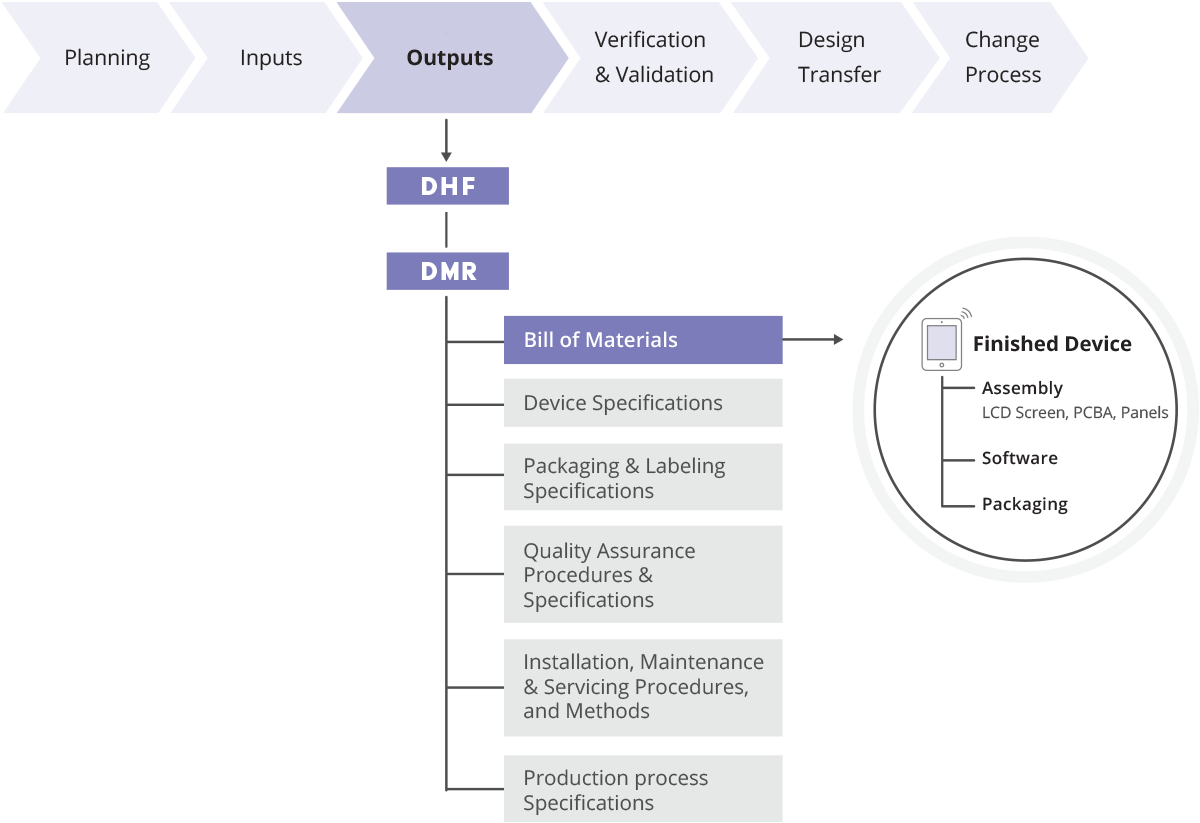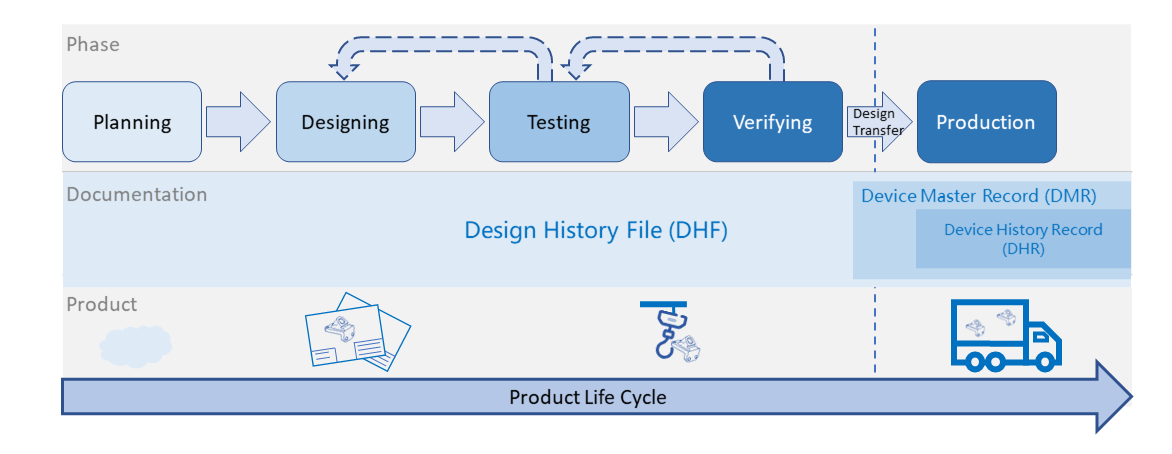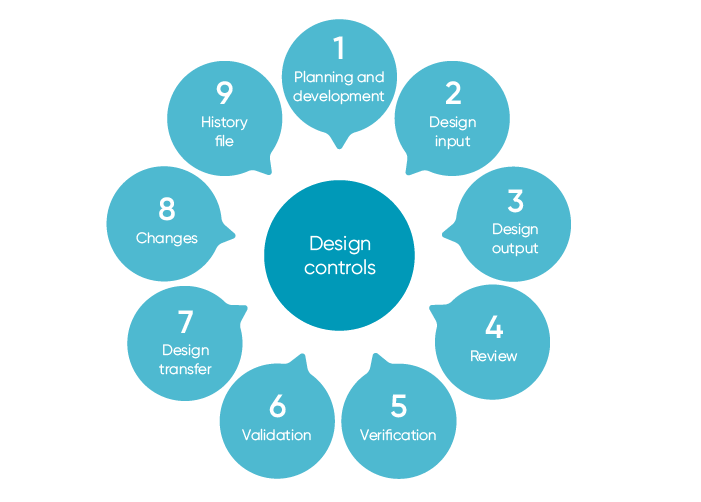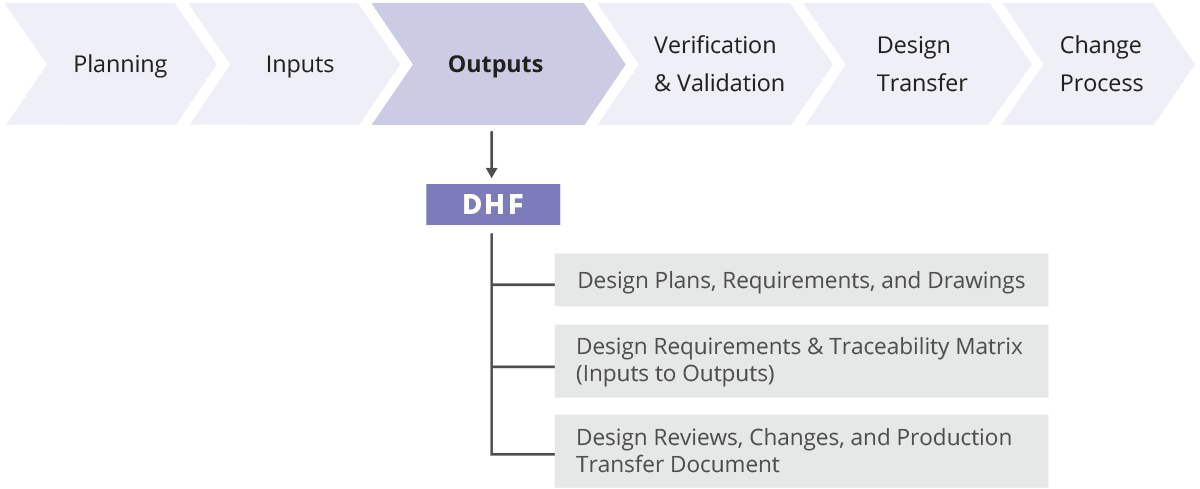
What is Design History File? Why it is Important for Medical Device Development | Medical Device - Johari Digital Healthcare Ltd.

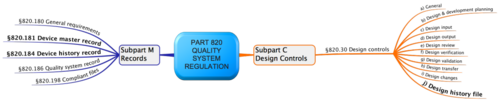
InstantGMP MD/PRO Electronic Device History File Software for Manufacturing Medical Devices in compliance with 21 CFR Part 820 (Quality System Regulation) - ppt download